Candida auris
A global health threat
Candida auris is an emerging yeast that has become one of the most concerning nosocomial fungal pathogens worldwide. Since its discovery in 2009, C. auris has spread rapidly across continents, causing difficult-to-control hospital outbreaks due to its high antifungal resistance and ability to persist on surfaces and medical equipment.
Recognized by the World Health Organization (WHO) as a critical priority fungal pathogen, C. auris poses major public health challenges because of its rapid transmission and misidentification by conventional diagnostic systems.
In March 2025, the UK Health Security Agency (UKHSA) released its first updated guidance on Candida auris since 2017, now officially reclassified as Candidozyma auris to reflect its distinct genetic profile. Published in The Lancet Microbe, this report underscores the growing clinical and environmental challenges posed by antifungal resistance worldwide. According to this data, detections in England nearly doubled from 93 in 2023 to 178 in 2024, with several outbreaks showing domestic transmission rather than imported cases.
Candida auris: a diagnostic challenge
Accurate identification of C. auris is essential for effective patient management and infection control. However, diagnosis remains difficult due to:
- Phenotypic misidentification with other Candida species in conventional systems.
- Multidrug resistance, especially to fluconazole, amphotericin B, and echinocandins.
- Environmental persistence and skin colonization, which require rapid and specific molecular detection to control outbreaks.
The new UKHSA guideline advises using MALDI-TOF, molecular assays, and WGS for accurate identification and outbreak monitoring. Screening is limited to high-risk or exposed patients, and confirmed cases should be isolated. This guidance also highlights antimicrobial stewardship to prevent antifungal resistance.
The value of molecular diagnosis
The UK Health Security Agency (UKHSA) now explicitly recommends replacing biochemical identification methods with molecular diagnostic assays or MALDI-TOF mass spectrometry for the accurate detection of Candidozyma auris in clinical and high-risk settings.
Among these, real-time PCR–based methods stand out for their superior sensitivity, specificity, and turnaround time, allowing laboratories to rapidly confirm species-level identification and detect both infections and colonization before they spread.
By delivering reliable results directly from clinical or environmental samples, PCR assays overcome the limitations of phenotypic methods, which often misidentify C. auris as other Candida species.
They allow laboratories to:
- Differentiate C. auris from closely related species.
- Detect infections and colonization early.
- Support infection control measures through active surveillance.

Our solution: quality assurance with AMPLIRUN®
While real-time PCR assays are now the reference standard for the rapid and accurate detection of Candidozyma auris, their reliability depends on the use of well-characterized molecular quality controls. At Vircell, we provide AMPLIRUN® Molecular Controls, a comprehensive range of reference materials specifically designed to support method validation, routine quality assurance, and external quality assessment (EQA).
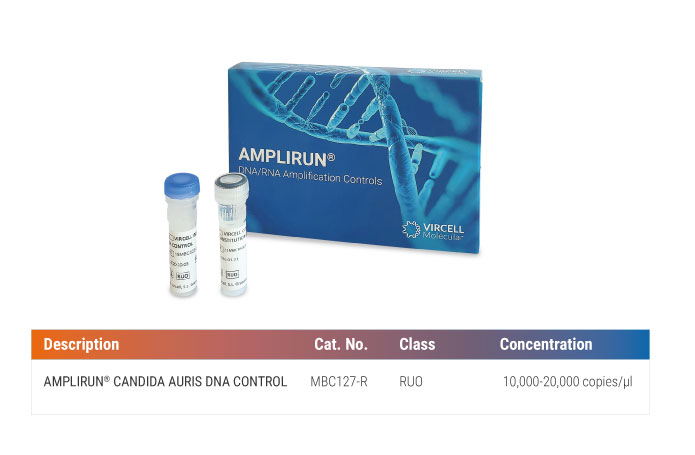
AMPLIRUN® CANDIDA AURIS DNA CONTROL (Ref. MBC127-R)
- Purified DNA of Candida auris for controlling nucleic acid amplification techniques.
- Complete microbial genome, any target can be amplified.
- Suitable for any molecular testing platform, including real-time and conventional PCR.
- Lyophilized presentation for long-term stability and easy transport.
- 30-month shelf life from the date of manufacture.
This control is ideal for verifying sensitivity, precision, and reproducibility in molecular assays targeting C. auris, supporting accurate and traceable results.
You can find more information on the AMPLIRUN® website
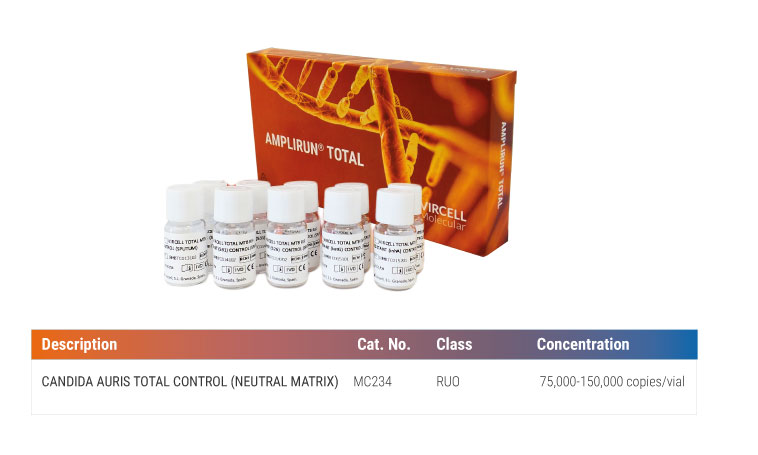
CANDIDA AURIS TOTAL CONTROL (NEUTRAL MATRIX) (Ref. MC234)
- Non-infectious, ready-to-use molecular control.
- Designed for full workflow verification of PCR assays, from extraction to detection.
- Suitable for performance evaluation, method validation, and external quality assurance (EQA).
- Formulated in a neutral matrix, compatible with most molecular platforms and workflows.
AMPLIRUN® ensures reliable, reproducible, and traceable results, supporting accurate detection of Candida auris in clinical and reference laboratories.
More information on our AMPLIRUN® website