Hepatitis E: The overlooked threat in liver infections
Hepatitis E virus: a silent global burden
Hepatitis E virus (HEV) is the leading cause of acute hepatitis globally. Though commonly associated with contaminated water and poor sanitation in developing countries, recent evidence shows increasing autochthonous infections in industrialized regions, highlighting its worldwide distribution.
How is HEV transmitted?
HEV is primarily transmitted through the fecal-oral route, often via contaminated water. However, unlike other hepatitis viruses, HEV has zoonotic potential and can infect a range of animal hosts, including pigs, deer, and wild boar. In developed countries, zoonotic transmission through undercooked pork or game meat is now considered a major route of infection.
Who is most vulnerable to hepatitis E?
The infection is especially dangerous in the following populations:
- Immunocompromised individuals, such as organ transplant recipients
- Pregnant women, particularly in the third trimester, with mortality rates up to 20–30%
- Individuals with hematologic conditions or chronic liver disease, where superinfection may be fatal
It is estimated that over 2 billion people have been infected with HEV globally, with 20 million new infections each year. This leads to approximately 3.3 million symptomatic cases and 57,000 deaths annually.¹
Despite these alarming figures, HEV remains underestimated, largely due to limited clinical awareness and the frequent misclassification of cases under other liver-related pathologies.
HEV testing recognized by WHO
In its 2023 update of the Essential Diagnostics List (EDL), the World Health Organization (WHO) has recognized the increasing impact of hepatitis E by officially including three diagnostic tests for HEV, including a rapid test for both clinical diagnosis and epidemiological surveillance.
This is a major step forward in raising awareness and improving access to HEV diagnostics, especially in low-resource settings and outbreak-prone regions.

The misdiagnosed threat: Why HEV often goes unnoticed
“The eyes can’t see what the mind doesn’t know.” In developed countries, hepatitis E is often misdiagnosed as drug-induced liver injury (DILI), especially among middle-aged and elderly patients. This lack of awareness contributes to delayed or incorrect treatments.
In these regions, HEV tends to cause more severe symptoms, with up to 15% of patients experiencing complications²,³. Fulminant hepatic failure occurs in approximately 8–11% of cases, and superinfection in chronic liver disease patients may result in mortality rates as high as 70%.
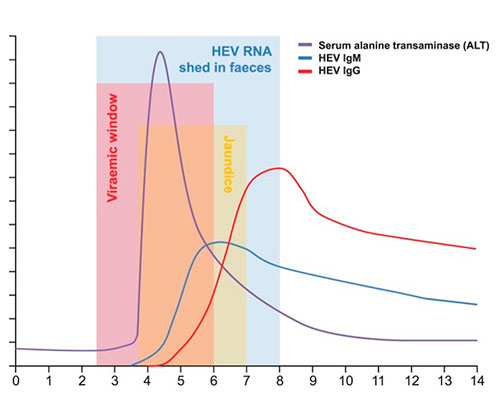
HEV incubation and diagnostic window
HEV has an incubation period of 2 to 8 weeks, during which time viraemia peaks. However, HEV RNA becomes undetectable in serum approximately 3 weeks after symptom onset, even though viral shedding in stool may continue for another 1–2 weeks.
🔬 Serological markers of HEV infection
- Anti-HEV IgM antibodies typically rise early and remain detectable for 3 to 12 months, indicating recent infection.
- This is followed by a strong IgG response, which peaks around 4 weeks after IgM and can remain detectable for more than a year, making IgG quantification crucial for distinguishing between acute and past infections.
✅ Available diagnostic methods for Hepatitis E:
- Serological testing: Detection of anti-HEV IgM and IgG antibodies
- Molecular detection: Identification of HEV RNA in serum or stool by RT-PCR, confirming active infection
Vircell Solutions for Hepatitis E
HEPATITIS E VIRCLIA® IgG MONOTEST (Quantitative)
- Detects IgG antibodies against HEV.
- Results expressed in IU/mL, standardized to the WHO reference reagent (NIBSC 95/584).
- Useful for monitoring prevalence in endemic areas and assessing immune response over time.
HEPATITIS E VIRCLIA® IgM MONOTEST (Capture)
- Detects IgM antibodies specific to HEV.
- High performance: 99% specificity, 92% sensitivity.
- A valuable tool for identifying primary infections during the acute phase.
HEPATITIS E VIRCLIA® IgG AVIDITY MONOTEST
- First CLIA monotest in the market for HEV avidity testing.
- Can be of aid for the serological diagnosis of primary infections.
- IgG avidity can distinguish recently adquired from resolved HEV GT3 infections in a clinical study. ⁵
International publications and scientific backing
Detection of HEV antibodies: chemiluminescence assay vs. ELISA
This study compares an automated chemiluminescent immunoassay (CLIA) by Vircell for the detection of anti-HEV IgM and IgG antibodies with a widely used ELISA test in clinical microbiology laboratories. It highlights the variability in sensitivity and specificity among commercial HEV assays and emphasizes the importance of reliable tools for accurate hepatitis E diagnosis.
Evaluation of a Novel CLIA Monotest Assay for the Detection of Anti-Hepatitis E Virus-IgG and IgM: A Comparison with a Line Blot and an ELISA
This study highlights the superior sensitivity of the VirClia® monotest (CLIA) compared to traditional ELISA and line blot assays. With excellent performance and a user-friendly format, it proves ideal for efficient hepatitis E diagnosis, especially in emergency and low-throughput settings.
Assessment of the hepatitis E Virclia® system for the detection of IgM and IgG antibodies against HEV
This study evaluates the performance of the VirClia® system for detecting anti-HEV IgM and IgG antibodies. As HEV is primarily transmitted via contaminated water or undercooked meat, accurate serological testing is essential. The research focuses on the sensitivity of this new method for diagnosing acute infection and identifying past exposure.
Comparative evaluation of two immunoassays for serological diagnosis of hepatitis E
This study compared the VirClia® chemiluminescent immunoassay (CLIA) with a manual enzyme immunoassay (EIA) for detecting anti-HEV IgM. In a panel of HEV-RNA positive and negative samples, VirClia showed higher sensitivity (94.1% vs. 76.5%) and comparable specificity (98.5%). The results highlight VirClia as a reliable alternative to conventional EIAs for acute HEV diagnosis.
Other resources
Do you have any question?
Bibliography
- Webb, G. W., & Dalton, H. R. (2019). Hepatitis E: an underestimated emerging threat. Therapeutic advances in infectious disease, 6, 2049936119837162.
- Dalton, H. R., et al. (2007). The role of hepatitis E virus testing in drug-induced liver injury. Alimentary pharmacology & therapeutics, 26(10), 1429–1435.
- Dalton, H. R., et al. (2008). Hepatitis E: an emerging infection in developed countries. The Lancet. Infectious diseases, 8(11), 698–709.
- Webb, G. W., & Dalton, H. R. (2019). Hepatitis E: an underestimated emerging threat. Therapeutic advances in infectious disease, 6, 2049936119837162
- Minosse, C., Lapa, D., Coppola, A., Rapagna, F., D'Offizi, G., Taibi, C., Lionetti, R., Capobianchi, M. R., McPhee, F., & Garbuglia, A. R. (2021). Anti-HEV IgG Avidity Testing: Utility for Diagnosing Acute and Resolved Genotype 3 Infections. Viruses, 13(2), 236



